Goal
We want to design a vapor-compression refrigeration cycle to absorb heat from a cool environment and reject it to a warm environment. The design is to be based upon the ideal vapor-compression refrigeration cycle, with four components: a cooler (where we reject the heat), a throttle, a heater (where we absorb the heat), and a compressor.
Basics of Vapor-Compression Refrigeration Cycles
The general idea
The challenge in refrigeration (and air conditioning, etc.) is to remove heat from a low temperature source and dump it at a higher temperature sink. Compression refrigeration cycles in general take advantage of the idea that highly compressed fluids at one temperature will tend to get colder when they are allowed to expand. If the pressure change is high enough, then the compressed gas will be hotter than our source of cooling (outside air, for instance) and the expanded gas will be cooler than our desired cold temperature. In this case, we can use it to cool at a low temperature and reject the heat to a high temperature.
Vapor-compression refrigeration cycles specifically have two additional advantages. First, they exploit the large thermal energy required to change a liquid to a vapor so we can remove lots of heat out of our air-conditioned space. Second, the isothermal nature of the vaporization allows extraction of heat without raising the temperature of the working fluid to the temperature of whatever is being cooled. This is a benefit because the closer the working fluid temperature approaches that of the surroundings, the lower the rate of heat transfer. The isothermal process allows the fastest rate of heat transfer.
More details
An ideal refrigeration cycle looks much like a reversed Carnot heat engine or a reversed Rankine cycle heat engine. The primary distinction being that refrigeration cycles lack a turbine, using a throttle instead to expand the working fluid. (Of course, a turbine could be incorporated into a refrigeration cycle if one could be designed to deal with liquids, but the useful work output is usually too small to justify the cost of the device.)
The cycle operates at two pressures, Phigh and Plow, and the statepoints are determined by the cooling requirements and the properties of the working fluid. Most coolants are designed so that they have relatively high vapor pressures at typical application temperatures to avoid the need to maintain a significant vacuum in the refrigeration cycle.
The T-s diagram for a vapor-compression refrigeration cycle is shown below.
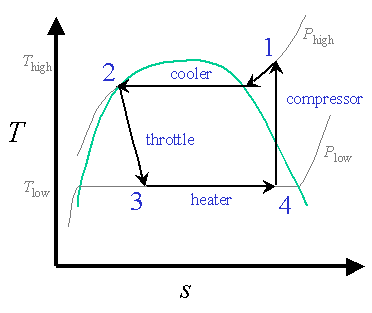
Figure 1: Vapor-Compression Refrigeration Cycle
T-s diagram
Below is a possible CyclePad design of a refrigeration cycle. The layout shown below is a clickable image. To jump to the part of this page that details the assumptions of a particular device or statepoint, just click on it.
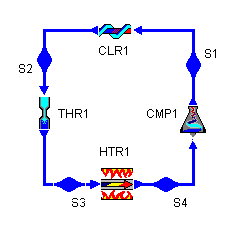 Figure 2: Basic refrigeration cycle layout |
|
Example Design Constraints
Cooling requirements
For purposes of illustration, we will assume that a refrigeration system used to cool air for an office environment. It must be able cool the air to 15.5°C (about 60°F) and reject heat to outside air at 32°C (90°F).
The working fluid
We have several working fluids available for use in refrigeration cycles. Four of the most common working fluids are available in CyclePad: R-12, R-22, R-134, and ammonia. (Nitrogen is also available for very low temperature refrigeration cycles.) We will choose R-22 for this example.
Description of Cycle Stages
We will examine each statepoint and component in the refrigeration cycle where design assumptions must be made, detailing each assumption. As we can see from the example design constraints, very few numbers need be specified to describe a vapor-compression refrigeration cycle. The rest of the assumptions are determined by applying reasoning and background knowledge about the cycle. The two principle numerical design decisions are determining Phigh and Tlow, at the cooler outletand the compressor inlet.
Cooler (Condenser) inlet (S1)This state need not involve any design decisions, but it may be important to come back here after the cycle has been solved and check that T2, which is the high temperature of the cycle, does not violate any design or safety constraints. In addition, this is as good a place as any to specify the working fluid.
Cooler (Condenser): Heat Rejection (CLR1)
The cooler (also known as the condenser) rejects heat to the surroundings. Initially, the compressed gas (at S1) enters the condenser where it loses heat to the surroundings. During this constant-pressure process, the coolant goes from a gas to a saturated liquid-vapor mix, then continues condensing until it is a saturated liquid at state 2. Potentially, we could cool it even further as a subcooled liquid, but there is little gain in doing so because we have already removed so much energy during the phase transition from vapor to liquid.
Cooler (Condenser) outlet (S2)
We cool the working fluid until it is a saturated liquid, for reasons stated above. An important design question arises at this state: how high should the high pressure of the cycle be?
We choose Phigh so that we can reject heat to the environment. Phigh is the same as P2, and P2 determines the temperature at state S2, T2. (T2 is just the saturation temperature at Phigh). This temperature must at least be higher than that of the cooling source, otherwise no cooling can occur.
However, if T2 is too high (that is, higher than the critical temperature TC for the working fluid), then we will be beyond the top of the saturation dome and we will loose the benefits of the large energy the fluid can reject while it is being cooled. Furthermore, it is often impractical and unsafe to have very high pressure fluids in our system and the higher P2 we choose, the higher T1 must be, leading to additional safety concerns. To find an applicable pressure, use the saturation tables to find a pressure which is somewhere between the saturation pressure of the warm air yet still in the saturation region.
For reference, TC for our four working fluids are given below.
| Critical Temperatures of some refrigerants | |
|---|---|
| substance | TC (°C) |
| R-12 (CCL2F2) | 111.85 |
| R-22 (CHCLF2) | 96.15 |
| R-134a (CF3CH2F) | 101.05 |
| ammonia (NH3) | 132.35 |
For our example using R-22, we must be able to reject heat to air that is 32°C. We can choose if T2 to be anywhere between that number and the 96°C TC. We'll choose it to be 40°C for now.
The figure above gives a general idea of the improvements we can expect with lower temperatures in the cooler. Keep in mind that the practical limitation here is heat transfer to the surrounding air. While lower temperatures will make the cycle more efficient theoretically, setting Thigh too low means the working fluid won't surrender any heat to the environment and won't be able to do its job.
Throttling (THR1)
The high-pressure, saturated liquid is throttled down to a lower pressure from state S2 to state S3. This process is irreversible and there is some inefficiency in the cycle due to this process, which is why we note an increase in entropy from state S2 to S3, even though there is no heat transfer in the throttling process. In theory, we can use a turbine to lower the pressure of the working fluid and thereby extract any potential work from the high pressure fluid (and use it to offset the work needed to drive the compressor). This is the model for the Carnot refrigeration cycle. In practice, turbines cannot deal with the mostly liquid fluids at the cooler outlet and, even if they could, the added efficiency of extracting this work seldom justifies the cost of the turbine.
Heater (Evaporator): Heat Absorption (HTR1)
The working fluid absorbs heat from the surroundings which we intend to cool. Since this process involves a change of phase from liquid to vapor, this device is often called the evaporator. This is where the useful "function" of the refrigeration cycle takes place, because it is during this part of the cycle that we absorb heat from the area we are trying to cool. For an efficient air conditioner, we want this quantity to be large compared to the power needed to run the cycle.
The usual design assumption for an ideal heater in a refrigeration cycle is that it is isobaric (no pressure loss is incurred from forcing the coolant through the coils where heat transfer takes place). Since the heating process typically takes place entirely within the saturation region, the isobaric assumption also ensures that the process is isothermal.
Compressor Inlet (S4)
Where do we want S4?
Typically, we want state S4 to be right at the saturated vapor side of the saturation dome. This allows us to absorb as much energy from the surroundings as possible before leaving the saturation dome, where the temperature of the working fluid starts to rise and the (now non-isothermal) heat transfer becomes less efficient.
Of course, we would get the same isothermal behavior if we were to start the compression before the fluid was completely saturated. Further, there would seem to be a benefit in that statepoint S1 (see Figure 1) would be closer to the saturation dome on the Phigh isobar, allowing the heat rejection to be closer to isothermal and, therefor, more like the Carnot cycle.
It turns out that, for increased efficiency, we can choose S4 such that S1 is on the saturation dome, instead of outside of it in the superheat region. Figure 4 shows the T-s diagrams for two refrigeration cycles, one where S4 is a saturated vapor and the other (in light green) where S4 has been moved further into the saturation dome to allow S1 to be a saturated vapor.
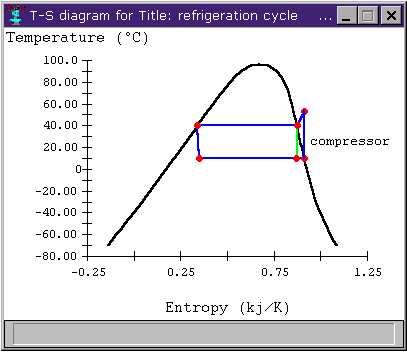
Figure 4: T-s diagram for different compressor conditions
The advantage in the second case is that we have reduced the compressor work. We have also reduced the heat transfer somewhat, but the reduced compressor work has a greater effect on the cycle's coefficient of performance. Figure 6 shows the cycle's COP versus the quality of S4. We note that the change in COP is noticable, but not terribly impressive.
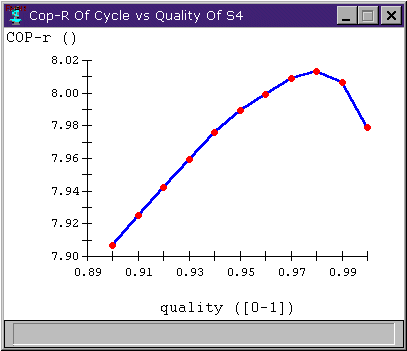
Figure 5: COP versus compressor inlet quality
However, in setting S4 below the saturated vapor line, we assume our compressor can work with fluid that is substantially liquid at statepoint S4. Since the liquid part of the fluid is incompressible, this is likely to damage the compressor. It is for this reason that we choose the inlet to the compressor to be completely saturated vapor, ensuring that the compressor can do its work entirely in the superheat region. When we are told we have compressors capable of dealing with fluids whose quality is slightly less than 100% (these are sometimes available), we can adjust the position of S4 to improve cycle efficiency.
How to choose Tlow
This brings us to another design issue: Now that we know that S4 is on the saturated vapor line, where on the line is it? In other words, how low can Tlow go?
Tlow occurs within the saturation dome, so it determines Plow as well. We know that Tlow must at least be cooler than the desired temperature of the stuff we wish to cool, otherwise no cooling will occur. An examination of the saturation tables for our refrigerants shows that setting Tlow at, for instance 15� C, still allows for fairly high pressures (4 to 7 atmospheres, typically). So, while this tells us how low Plow must be, it does not tell us how low it can be.
There are several major practical considerations limiting Plow. Fundamentally, we must concern ourselves with the properties of our working fluids. Examination of the saturation table for R-22 shows that at atmospheric pressure, the saturation temperature is already very cold (about -40°C). For small-scale air-conditioning applications, we have no desire to create a stream of extremely cold air, both due to safety concerns and because cold air holds very little moisture and can be uncomfortably dry. For larger-scale applications, this is less of a concern because we can always mix the cold, dry air with warmer, wetter air to make it comfortable.
Another hardware consideration is that it is fairly difficult to maintain a very low-pressure vacuum using the same compressor that will achieve high pressure at its outlet. Choosing a Tlow that results in a Plow of 0.1 atmospheres is probably not practical if we intend to have Phigh up near 10 atmospheres.
This brings us to the other reason we cannot make Tlow too small. Examining Figure 1 again, we see that the lower Plow is, the further out to the right (higher entropy) the saturated vapor will be at statepoint S4. Statepoint S4 has the same entropy as S1, and the further to the right S1 is along the Phigh pressure isobar, the hotter S1 must be. This high temperature is undesirable from both efficiency and safety standpoints.
The figure below shows the relationship between Tlow and the cycle's coefficient of performance (COP). We note that the higher Tlow, the better the COP. The practical limit on Tlow is heat transfer rate in the evaporator; having Tlow too close to the temperature of the stuff we wish to cool results in low heat transfer rates.
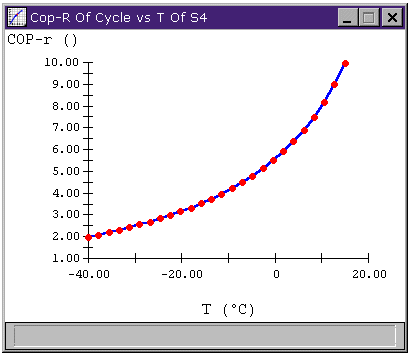
Figure 6: Vapor-Compression Refrigeration Cycle
COP versus Tlow
So, ultimately, we want a low pressure such that its saturation temperature is below the desired cool air temperature but high enough that the temperature at state one is not too hot. For our example, where we need to cool air down to 15.5°C, we will choose Tlow to be 10°C.
Compressor (COMP1)
Ideal compressors are like ideal pumps, adiabatic and isentropic. We also note that the compressor is the only device in the system that does work to the fluid. For an efficient air conditioner, we want this quantity to be small.
Great Design, very well explained.
ReplyDeleteCheck out our free online heat and refrigeration load calculator at enthalpya.com